
The continuous monitoring of neonate vital signs is standard practice in the NICU. But even though preterm and critically ill newborns often experience abnormalities in brain activity that can indicate serious conditions, many NICUs still do not have the capacity to continuously monitor neurological status. As survival rates increase, more emphasis is being placed on improving long-term health outcomes for these infants, and neonatologists worldwide increasingly recommend the use of amplitude-integrated electroencephalography (aEEG) to monitor neonate brain function in the NICU continuously.
aEEG is a streamlined diagnostic technology that offers an accessible way to monitor neonate electrocortical activity at the bedside. Real-time, easy-to-understand data from aEEG monitoring can immediately capture abnormalities in between diagnostic workups and/or when EEG is unavailable. For these reasons and more, the use of aEEG is rapidly transforming how healthcare professionals diagnose and treat neonatal brain disorders, helping minimize the risk of further brain damage, improve long-term health outcomes, and provide more accurate quality-of-life assessments.
In this article, we’ll examine the rising use of aEEG in the NICU, why aEEG data is helpful throughout the full spectrum of neonate healthcare, and how this advanced technology supports multidisciplinary care.
aEEG is extremely useful in the NICU because it delivers information to the NICU staff right at the bedside, providing a simplified yet meaningful visual display of a neonate’s brain activity. In addition, neonatologists can read and understand aEEG without the extensive training required for EEG interpretation. This helps support multidisciplinary care by providing data that allows neonatologists to submit more informed requests for full EEG and/or pediatric neurology consultations, improving the quality of referrals to neurologists and other specialists.
Less invasive than standard EEG monitoring, aEEG works by applying three to five electrodes on a newborn’s scalp. The electrodes immediately begin delivering continuous, easy-to-understand data. With aEEG, NICU staff can more closely monitor information on the effectiveness and potential impacts of sedation, anticonvulsive therapy, hypothermia and other treatments, and ventilation. aEEG is especially useful for detecting subclinical seizures and for providing data on sleep/waking cycles, two important predictors of health outcomes during the perinatal period. Overall, empirical data and research prove that early recovery of aEEG abnormalities correlates with better neurodevelopmental outcomes.¹
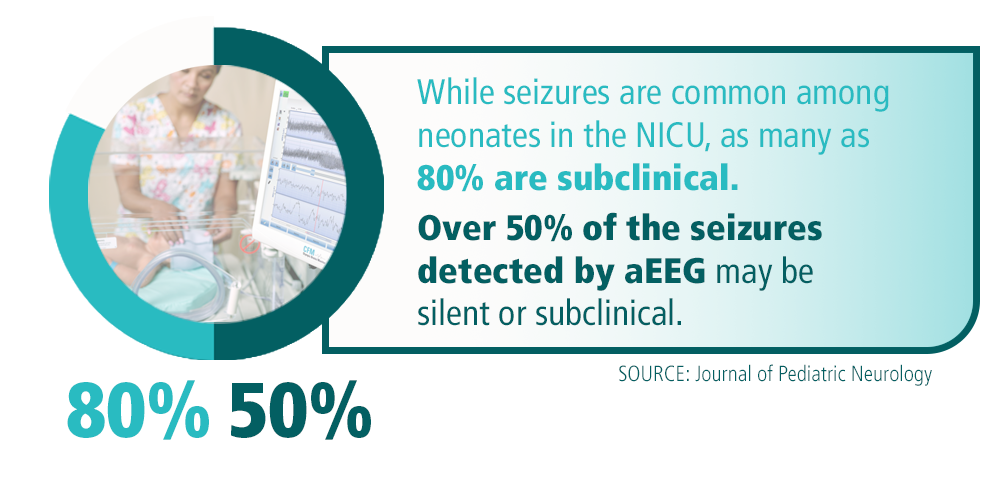
Seizures can be detrimental to the immature brain of a newborn and negatively impact long-term neurological outcomes. While seizures are common among neonates in the NICU (over two-thirds occur within the first week of life), as many as 80% are subclinical², intensifying the need for continuous brain monitoring.
 Seizures are an important indicator of potential central nervous system diseases, and data from aEEG offers a pathway for neonatologists to diagnose conditions earlier and more accurately.
Seizures are an important indicator of potential central nervous system diseases, and data from aEEG offers a pathway for neonatologists to diagnose conditions earlier and more accurately.
A study reported in the Journal of Pediatric Neurology states that over half of the seizures detected by aEEG technologies may be silent or subclinical, noting that aEEG can…” provide important information concerning neurologic status and help to confirm or refute the presence of seizures in clinically suspected cases and detect infants with silent seizures.³”
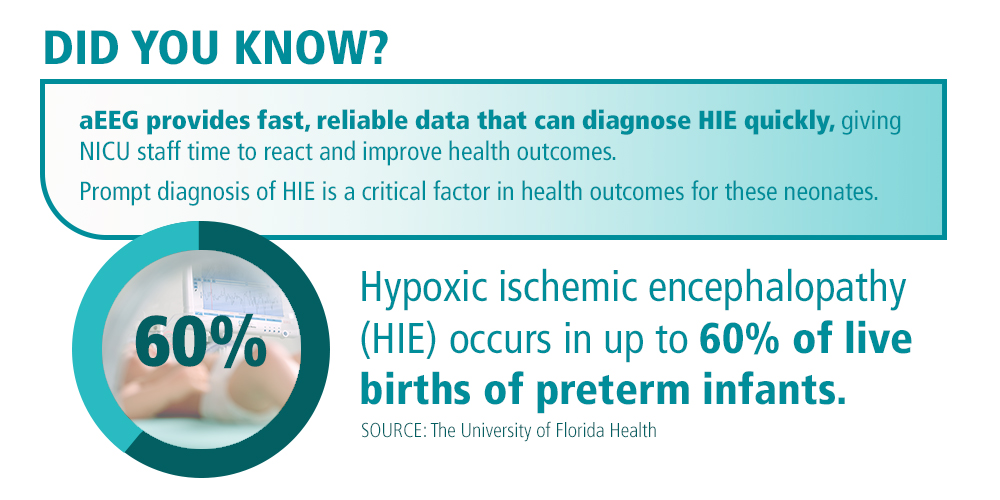
As neonatal intensive care technologies advance, neonatologists have focused on finding more ways to decrease the potential of long-term disability for at-risk newborns.. Of particular concern is hypoxic ischemic encephalopathy (HIE), which, according to researchers at the University of Florida, occurs in up to 60% of live births of preterm infants. HIE occurs in full-term infants at a rate of approximately 3-20 per 1000 live births.4
Post-diagnosis, continuous brain monitoring of aEEG for newborns with HIE offers several benefits, including:
Evidence shows significant benefits of using aEEG in the NICU, beyond HIE. Download a compilation of case studies to learn how brain monitoring helps to improve patient outcomes and prognosticate the future for neonates.
While aEEG became standard for neonates with HIE many years ago, it has increasingly shown to improve outcomes for many other conditions for several reasons:
Additionally, aEEG can predict long-term neurodevelopmental outcomes by identifying the extent of brain damage and monitoring changes in brain activity over time. The benefits of aEEG for newborn prognostication include accurate diagnosis of the severity of brain injury, early detection of brain damage, and prediction of long-term neurodevelopmental outcomes. This information is valuable for NICU staff tasked with counseling families and helping them better plan for long-term care.
For all neonates in the NICU, aEEG is a non-invasive way to continuously monitor neonate brain function, guide clinical decision-making, and potentially reduce the need for other therapies.
Overall, aEEG is a valuable tool that aids in diagnosing and managing neonatal brain disorders and improves health outcomes for at-risk newborns. Advancements in aEEG technology have helped overcome many perceived challenges, including the misconception that aEEG requires extensive specialized training to interpret results accurately. Today, neonatologists are encouraging the use of aEEG in the NICU as a less-invasive, continuous way to better understand the brain activity of preterm and critically ill newborns.
aEEG provides vital data that completes the whole picture of neonate health through:
Perhaps most importantly, aEEG helps with better prognostication, helping neonatologists determine the best treatment paths and provide better counsel for families often facing difficult decisions.
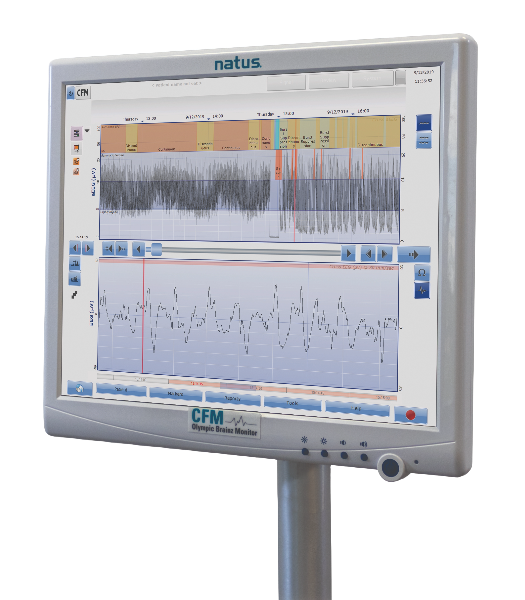
Always accessible in the NICU, the Olympic Brainz Monitor (OBM) was designed for neonatologists and made for NICU staff to recognize, at a glance, potential subclinical seizures and changes in the newborn’s neurological status. With the OBM from Natus, physicians and other staff can identify problems in real-time, creating more informed referrals and ultimately improving health outcomes for critically ill and preterm newborns. The OBM color-coded display simplifies the interpretation of brain activity, allowing healthcare professionals to easily identify changes in brain activity without extensive training. Learn why the OBM is the leader in aEEG in the NICU.
SOURCES
1. Singh A, Saluja S, Kler N, Garg P, Soni A, Thakur A. Amplitude integrated EEG: how much it helps in prognostication in neonatal encephalopathy? J Matern Fetal Neonatal Med. 2022 Dec;35(25):7748-7755. doi: 10.1080/14767058.2021.1937104. Epub 2021 Jun 13. PMID: 34121586.
2. Shany E, Khvatskin S, Golan A, Karplus M. Amplitude-integrated electroencephalography: a tool for monitoring silent seizures in neonates. Pediatr Neurol. 2006 Mar;34(3):194-9. doi: 10.1016/j.pediatrneurol.2005.06.018. PMID: 16504788.
3. Westhall, E., Rossetti, A. O., van Rootselaar, A. F., Wesenberg Kjaer, T., Horn, J., Ullén, S., Friberg, H., Nielsen, N., Rosén, I., Åneman, A., Erlinge, D., Gasche, Y., Hassager, C., Hovdenes, J., Kjaergaard, J., Kuiper, M., Pellis, T., Stammet, P., Wanscher, M., Wetterslev, J., … TTM-trial investigators (2016). Standardized EEG interpretation accurately predicts prognosis after cardiac arrest. Neurology, 86(16), 1482–1490. https://doi.org/10.1212/WNL.0000000000002462
4.Hypoxic-Ischemic Encephalopathy (HIE) – UF Health. ufhealth.org. Accessed July 12, 2023. https://ufhealth.org/conditions-and-treatments/hypoxic-ischemic-encephalopathy-hie
5. Nathalie H.P. Claessens, Lotte Noorlag, Lauren C. Weeke, Mona C. Toet, Johannes M.P.J. Breur, Selma O. Algra, Antonius N.J. Schouten, Felix Haas, Floris Groenendaal, Manon J.N.L. Benders, Nicolaas J.G. Jansen, Linda S. de Vries, Amplitude-Integrated Electroencephalography for Early Recognition of Brain Injury in Neonates with Critical Congenital Heart Disease, The Journal of Pediatrics, Volume 202, 2018, Pages 199-205.e1, ISSN 0022-3476, https://doi.org/10.1016/j.jpeds.2018.06.048. (https://www.sciencedirect.com/science/article/pii/S0022347618308734)
044666 RevA