Over the years, newborn hearing screening programs have advanced to screen for congenital hearing loss as early as the first day of a baby’s life. Yet, some conditions are still missed during newborn screening that is performed with OAE alone. Auditory Neuropathy Spectrum Disorder (ANSD) will pass a screen as normal Otoacoustic Emissions (OAE), as these infants have normal cochlear function. Using Automated Auditory Brainstem Response (AABR) to screen the entire hearing pathway can help identify auditory nerve abnormality.
This article highlights the importance of considering changing approaches newborn hearing screening programs to optimize the use of AABR as a primary screening protocol, with OAE as a secondary screening recommendation in the absence of clear response from AABR.
Auditory neuropathy spectrum disorder (ANSD) can present at any age and affects approximately one to three children per 10,000 births. ANSD is an auditory dysfunction where the ear receives sounds, but damage at some point along the auditory nerve results in the child not being able to process sound normally. The level of hearing loss can vary from mild to profound, but there is often a mismatch between the hearing threshold and speech perception abilities. ANSD may result from both syndromic and non-syndromic genetic anomalies, environmental causes, as well as structural anomalies1.
According to Sininger & Oba’s study from 20012, speech perception in ANSD patients is considerably worse than can be predicted from a pure-tone audiogram. As such, the audiogram is a limited tool for diagnosing this auditory dysfunction that can have a considerable impact on a child’s speech and language development.
Otoacoustic emissions (OAEs) measure the function of outer hair cells in the cochlea, which are responsible for sound stimulus amplification. While the child is quiet and settled or asleep, a small probe is placed in the ear canal to deliver a click or tone sound and a microphone within the probe measures the acoustic response from the cochlea to that sound stimulus. In most cases of childhood hearing loss, the outer hair cells are damaged or absent. But as ANSD is characterized by a normal cochlear function, OAEs and/or cochlear microphonic (CM) will be present at some point and the screening of the outer hair cells with OAE will pass as normal.
Automated auditory brainstem response (AABR) examines how well the auditory nerve sends sound from the inner ear to the brainstem by screening the entire hearing pathway. This screening method can determine if the brain is receiving information in a clear way. Ear couplers are placed over the infant’s ear, and a sound stimulus of 35dBnHL or 40dBnHL is delivered into the ear canal. Each click evokes a series of identifiable brain waves from the infant’s auditory brainstem which are measured by electrode sensors placed on the child’s forehead, nape of neck and shoulder. In ANSD patients, ABR will be absent or severely abnormal when maximal stimulus is used. This shows poor responses from the auditory nerve, the inner hair cells or the basal ganglia.
Accordingly, normal OAE and abnormal AABR results must be present to diagnose infants with ANSD. Many screening programs around the world are not suited to identify ANSD in healthy babies because they rely exclusively on OAE as a first-step screen, which are typically normal in subjects with ANSD. According to JCIH position statement (2019)3, ideally, newborn hearing screening should be performed before one month of age, the diagnosis should be verified by three months by an audiologist and intervention should begin by six months. All neonatal intensive care unit (NICU) infants should be screened with AABR as they are at a higher risk of ANSD and other auditory nerve pathologies.

ANSD can affect people of all ages, from birth to adulthood. Initially, ANSD was thought to be rare. But the current prevalence estimates in children show that one to three children per 10,000 births are affected by ANSD.
The prevalence of ANSD in the well-baby population with confirmed congenital hearing loss varies between 6.5% (Boudewyns et al4) and up to 10%5. The incidence is higher in the NICU graduate population being up to 10-15% with the NHS Newborn Hearing Screening Program (NHSP) Evaluation from 20046 showing that 0.2% of babies in neonatal intensive care units suffer from ANSD (Heather 2014) and demonstrating that about 50% of ANSD children have spent time in the NICU7. This leaves 50% of ANSD children who have NOT spent time in the NICU – and may be missed if they do not receive AABR screening at birth.
ANSD can be inherited or acquired7. It is possible to explore etiological factors of ANSD in well-baby populations. Some studies have found that ANSD can be caused by cochlear nerve deficiency (Buchman et al 20068) as well as tumors or cysts (Boudewyns et all 2018).
In addition, several genetic mutations have been reported9 in children with ANSD and include:
Genetic screening of infants with ANSD can provide ethical issues and informed consent should be taken from parents prior to testing. However, genetic and cytomegalovirus testing can help discover many late-onset progressive hearing loss cases as well as the otoferlin, pejvakin and other mutations in healthy babies. Genetic screening can also provide etiological information for different medical prognosis and syndromes, such as Friedreich’s Ataxia and Charcot-Marie-Tooth Syndrome. In addition, the genetic cause of ANSD will indicate the need for early trial of amplification and cochlear implants
Due to the high incidence of ANSD and other hearing deficits in infants in the NICU, it is recommended that these infants are primarily screened with AABR. Those who refer on the AABR screen should have a second screen with OAE to determine if the OAE is present – this will help the diagnostic audiologist with further investigations. In this population, the most commonly known risk factors for ANSD are:
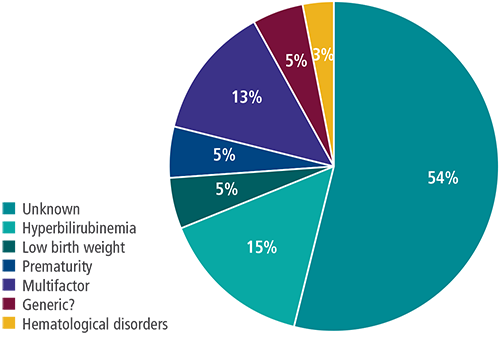
However, it is known that ANSD is present in children who have not been admitted to the NICU, and common risk factors cannot always be attributed as the cause. Gökdoğan et al (2016)10 demonstrated in their population that the etiology of the subject group showed 54% of infants with ANSD had unknown etiology and were not at-risk infants who would be screened with AABR as a first consideration.
Since the clinical presentation of ANSD varies, treatments are chosen according to the site in the auditory pathway affected, the severity of the ANSD and clinical deficits. Many ANSD patients will experience a spontaneous recovery, while others can improve speech understanding difficulties with amplification or assistive listening devices, such as Frequency Modulation (FM) systems that can reduce background noise and enhance a speaker’s voice or hearing aids that amplify sounds coming into the ear.
In most cases, a successful treatment for ANSD is a cochlear implant that stimulates the auditory nerve directly. Even if amplification is helping the child show progress in speech and language development, they can still benefit from a cochlear implant to improve sound awareness. If cochlear implants or hearing aids are not sufficient, it is also possible to manage ANSD with auditory stimulation and visual communication, including Cued Speech or sign language.
In the past, it may not have been cost effective to change approaches to newborn hearing screening programs. However, with the enhanced knowledge of ANSD, it might be time to review the cost-effectiveness based on equipment cost, time consumption and workforce.
OAE testing can be performed quickly with one single-use disposable ear tip per screening, resulting in lower consumable cost for the screening program. In comparison, it takes a few minutes to prepare the infant for AABR screening and to measure the brainstem responses in both ears simultaneously. Most AABR technologies use disposable, single-use sensors and ear couplers. Though this has a higher cost per screening than OAE testing, AABR testing results in a lower refer rate and fewer re-screenings, which reduces total program costs and loss to follow up, raising awareness regarding screening method trade-offs for capturing permanent hearing loss in healthy infants.
The main reason to include AABR as part of the hearing screening program lies in the fact that AABR technology has the highest sensitivity (lowest false negatives) and highest specificity (lowest false positives) as compared to OAE screening11 and is the only way of identifying infants who may have ANSD and require further diagnostic evaluation. Therefore, long term cost effectiveness with early identification (using AABR) and intervention in infants with ANSD will reduce the long-term health, education and social costs related to a child with hearing deficits (WHO 2017).
References:
1. De Siati, R.D.; Rosenzweig, F.; Gersdorff, G.; Gregoire, A.; Rombaux, P.; Deggouj, N. Auditory Neuropathy Spectrum Disorders: From Diagnosis to Treatment: Literature Review and Case Reports. J. Clin. Med. 2020, 9, 1074. https://doi.org/10.3390/jcm9041074
2. Sininger, Y. & Oba, Sandra. (2001). Patients with auditory neuropathy: who are they and what can they hear? In: Sininger Y, Starr A, eds. Auditory Neuropathy: A New Perspective on Hearing Disorders. San Diego: Singular Publishing. 15-35.
3. Year 2019 Position Statement: Principles and Guidelines for Early Hearing Detection and Intervention Programs. The Journal of Early Hearing Detection and Intervention 2019; 4(2): 1–44
4. Boudewyns, A., Declau, F., van den Ende, J. et al. Auditory neuropathy spectrum disorder (ANSD) in referrals from neonatal hearing screening at a well-baby clinic. Eur J Pediatr 175, 993–1000 (2016). https://doi.org/10.1007/s00431-016-2735-5
5. Rance, G., Starr, A. (2017). Auditory neuropathy spectrum disorder. In Tharpe, A. M., Seewald, R. (Eds.), Comprehensive handbook of pediatric audiology (2nd ed., pp. 227–246). Plural Publishing.
6. Rance G, Beer DE, Cone-Wesson B, et al. (1999). Clinical findings for a group of infants and young children with auditory neuropathy. Ear Hear 21: 238–252
7. Lindsey, Heather Making Sense of Auditory Neuropathy Spectrum Disorder, The Hearing Journal: June 2014 – Volume 67 – Issue 6 – p 8,9,12 doi: 10.1097/01.HJ.0000451359.32725.3f
8. Buchman CA, Roush PA, Teagle HF, Brown CJ, Zdanski CJ, Grose JH. Auditory neuropathy characteristics in children with cochlear nerve deficiency. Ear Hear. 2006 Aug;27(4):399-408. doi: 10.1097/01.aud.0000224100.30525.ab. PMID: 16825889
9. Guidelines for Aetiological Investigation into Auditory Neuropathy Spectrum Disorder in Children and Young Adults https://www.baap.org.uk/uploads/1/1/9/7/119752718/guidelines_for_ansd_final_version.pdf
10. Çağıl Gökdoğana, Şenay Altınyaya, Bülent Gündüza, Yusuf Kemal Kemaloğlub, Yıldırım Bayazıtb, Kemal Uygurb. Management of children with auditory neuropathy spectrum disorder (ANSD). Braz J Otorhinolaryngol. 2016;82:493-9
11. Katarzyna E et al, Universal newborn hearing screening: methods and results, obstacles, and benefits. Pediatr Res 81, 415–422 (2017)
041189 RevA
